- Souvenaid® is an evidence based medical drink supported by 20 years of research and proven to support memory function in patients with mild cognitive impairment and mild Alzheimer’s disease.1-4
- Souvenaid® contains the active component Fortasyn Connect, a unique combination of nutrients formulated to support synapse formation and function in Alzheimer’s disease.5
- Souvenaid® is available in either liquid or powder format in 6 flavours and should be taken once daily for a period of at least 6 months.1-3
- Souvenaid® can be purchased through pharmacies or via Souvenaid Connections, which is a patient support program run by Nutricia offering support, services and discount pricing.

Synapse loss is an early event in the progression of Alzheimer’s disease
Alzheimer’s disease is the most common form of dementia. It is a progressive disease that is characterised by symptoms such as impairments in memory function, language, thought and problem-solving. In the earliest stages of the disease, memory loss is the most commonly reported symptom.
Alzheimer’s disease is characterised by beta-amyloid plaques, neurofibrillary tangles, neurone loss and synapse loss.6-9 Compared with the other hallmarks of Alzheimer’s disease, synapse loss correlates best with impaired memory, and is believed to occur early in the disease process, even before the onset of clinical Alzheimer’s disease symptoms.7-9
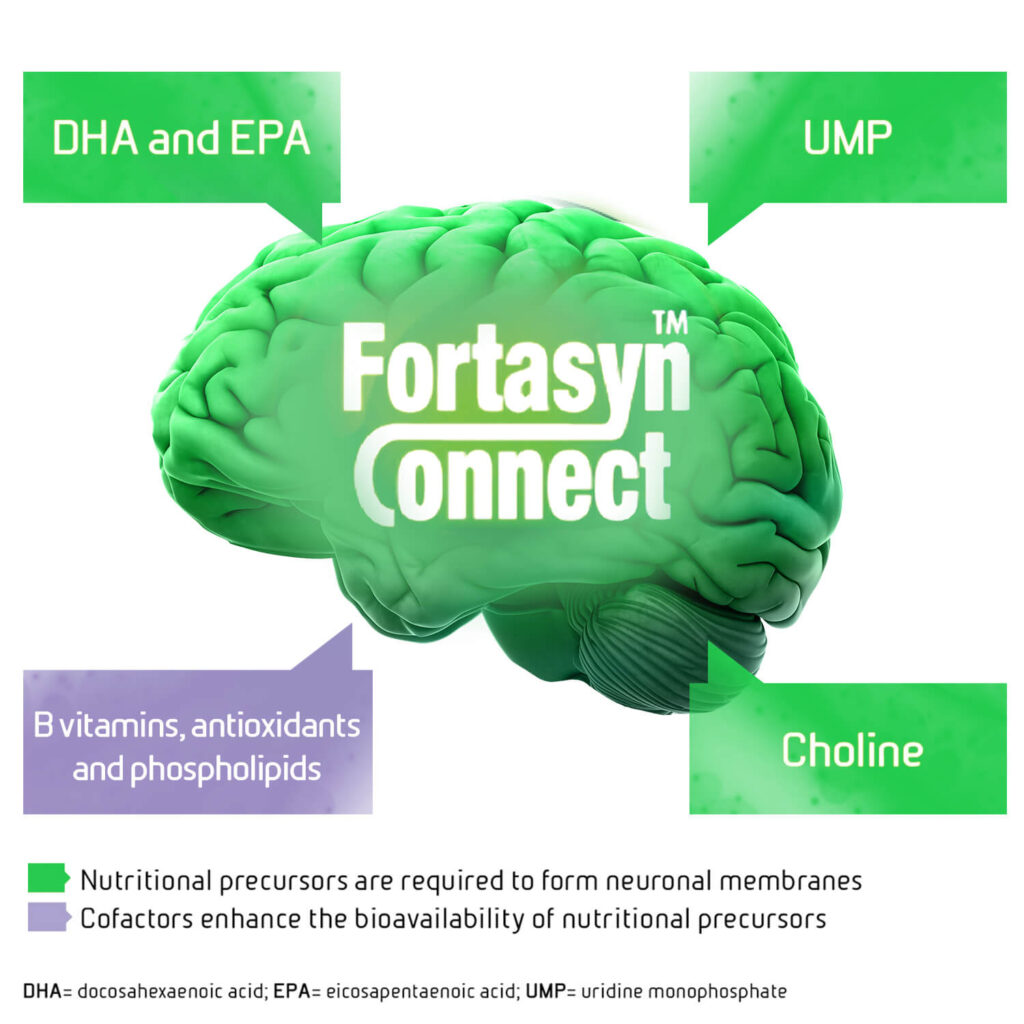
Souvenaid (Fortasyn Connect) – formulated to support synapse formation and function5
Synapse formation depends on the production of neuronal membranes, which primarily consist of phospholipids.10-11 Phosphatidylcholine is the most abundant phospholipid in the brain, which is generated primarily via the Kennedy pathway. The production of phospholipids via this pathway is positively affected by the availability of the required nutritional precursors uridine monophosphate, choline and omega 3 fatty polyunsaturated fatty acids.11
Souvenaid (Fortasyn Connect) is the unique combination of these nutritional precursors along with cofactors.5 These components work together to enhance phospholipid synthesis via the Kennedy pathway. The increased phospholipid synthesis supports the formation of neuronal membranes and synapses.11
Souvenaid is supported by 20 years of research1-4
The transition of Souvenaid from research bench to real-world patients is based on decades of research conducted by the MIT and other international institutions.1-4,12-22
Clinical trials have evaluated Souvenaid in early Alzheimer’s disease and demonstrated clinically meaningful benefits for patients.1-4
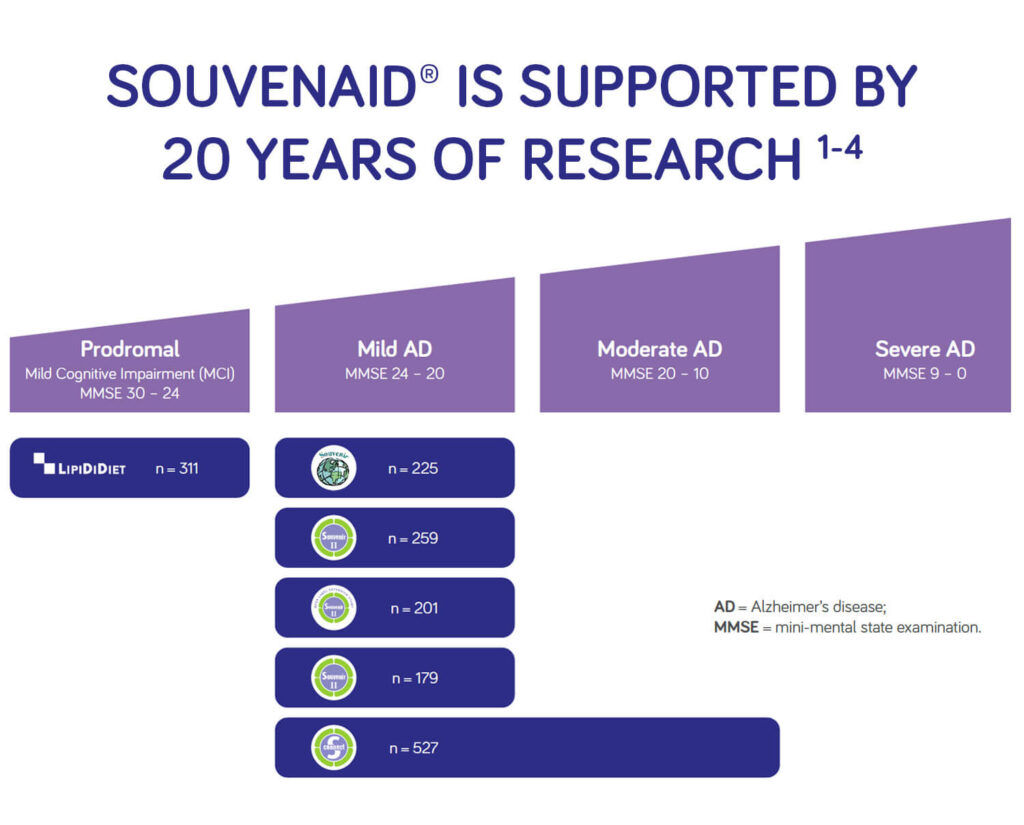
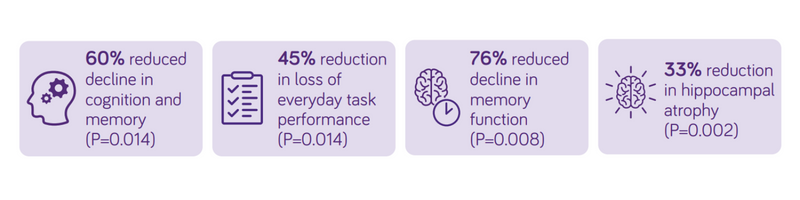
The first clinical trials with Souvenaid showed encouraging effects on memory performance in patients with mild AD dementia.1,2 Souvenaid was associated with a statistically significant improvement in memory performance in patients with mild and very mild AD dementia, observed over 12 weeks in Souvenir I and 24 weeks in Souvenir II 1,2 and patients continued to exhibit improved memory for up to 48 weeks.23
Based on the results of Souvenir I and II, an independent European Commission funded a new trial focusing on the longer-term use of Souvenaid for memory and cognitive function in the very early stages of Alzheimer’s disease – known as “prodromal AD” or “mild cognitive impairment” (MCI). With patients potentially followed up to 8 years, the LipiDiDiet study is the longest intervention trial in MCI.3
At 3 years the trial concluded that, in people with prodromal Alzheimer’s disease/MCI, the daily consumption of Souvenaid showed significant positive results (slowed decline) in cognitive function, thinking skills, memory, and brain atrophy.3
How can my patient purchase Souvenaid®?
Souvenaid® is available in either liquid or powder format and can be purchased from local pharmacies or directly through NutriciaStore.

Souvenaid® liquid

Souvenaid® Powder
Is there support available for patients on Souvenaid®?
Souvenaid® Connections is a comprehensive care program for patients with MCI or Mild Alzheimer’s disease and their carers.
Our goal is to provide support for every patient and carer along their treatment journey and help them achieve positive health outcomes by taking Souvenaid® regularly.
- Exclusive discounts on Souvenaid®
- Support from Souvenaid®’s specialist team
You may register your patients via the online patient registration form below
Register here
Souvenaid® is a food for special medical purposes for the dietary management of early Alzheimer’s disease and must be used under medical supervision.
For healthcare professionals only.
- Scheltens P et al. Efficacy of a medical food in mild Alzheimer’s disease: A randomized, controlled trial. Alzheimers Dement 2010; 6(1): 1–10.e1.
- Scheltens P et al. Efficacy of Souvenaid in mild Alzheimer’s disease: results from a randomized, controlled trial. J Alzheimers Dis 2012; 31(1): 225–36.
- Soininen H, et al. 36-month LipiDiDiet multinutrient clinical trial in prodromal Alzheimer’s disease. Alzheimer’s Dement. 2021;17:29–40.
- Shah RC et al. The S-Connect study: results from a randomized, controlled trial of Souvenaid in mild-to-moderate Alzheimer’s disease. Alzheimers Res Ther 2013; 5: 59.
- Sijben J et al. (2011). A multinutrient concept to enhance synapse formation and function: Science behind a medical food for Alzheimer’s disease. OCL. 18. 267-270.
- Terry RD, et al. Physical basis of cognitive alterations in Alzheimer’s disease: Synapse loss is the major correlate of cognitive impairment. Ann Neurol 1991;30:572-580.
- 7. Terry RD. Alzheimer’s disease and the aging brain. J Geriatr Psychiatry Neurol 2006;19:125-128.
- Scheff SW, et al. Hippocampal synaptic loss in early Alzheimer’s disease and mild cognitive impairment. Neurobiol Aging 2006;27:1372-1384.
- Sperling RA, et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:280-292.
- Yi JJ and Ehlers MD. Ubiquitin and protein turnover in synapse function. Neuron. 2005;47:629-632.
- Kennedy EP and Weiss SB. The function of cytidine coenzymes in the biosynthesis of phospholipides. J Biol Chem. 1956;222:193-21]Kennedy EP, Weiss SB (1956) The function of cytidine coenzymes in the biosynthesis of phospholipides. J Biol Chem 222, 193-214
- Wurtman RJ, et al. Synaptic proteins and phospholipids are increased in gerbil brain by administering uridine plus docosahexaenoic acid orally. Brain Res. 2006;1088:83-92.
- Cansev M, et al. Restorative effects of uridine plus docosahexaenoic acid in a rat model of Parkinson’s disease. Neurosci Res.2008;62:206-209.
- Sakamoto T, et al. Oral supplementation with docosahexaenoic acid and uridine-5′-monophosphate increases dendritic spine density in adult gerbil hippocampus. Brain Res. 2007;1182:50-59.
- Holguin S, et al. Chronic administration of DHA and UMP improves the impaired memory of environmentally impoverished rats.Behav Brain Res. 2008;191:11-16.
- Holguin S, et al. Dietary uridine enhances the improvement in learning and memory produced by administering DHA to gerbils. FASEB J. 2008;22:3938-3946.
- Cansev M, et al. Giving uridine and/or docosahexaenoic acid orally to rat dams during gestation and nursing increases synaptic elements in brains of weanling pups. Dev Neurosci. 2009;31:181-192.
- Cansev M and Wurtman RJ. Chronic administration of docosahexaenoic acid or eicosapentaenoic acid, but not arachidonic acid, alone or in combination with uridine, increases brain phosphatide and synaptic protein levels in gerbils. Neuroscience. 2007;148:421-431.
- Wang L, et al. Dietary supplementation with uridine-5′-monophosphate (UMP), a membrane phosphatide precursor, increases acetylcholine level and release in striatum of aged rat. Brain Res. 2007;1133:42-48.
- Richardson UI, et al. Stimulation of CDP-choline synthesis by uridine or cytidine in PC12 rat pheochromocytoma cells. Brain Res. 2003;971:161-167.
- Richardson UI and Wurtman RJ. Polyunsaturated fatty acids stimulate phosphatidylcholine synthesis in PC12 cells. Biochim Biophys Acta. 2007;1771:558-563.
- Van Wijk N, et al. Plasma choline concentration varies with different dietary levels of vitamins B6, B12 and folic acid in rats maintained on choline-adequate diets. Br J Nutr. 2012;107:1408-12
- Rikkert O et al. Tolerability and safety of souvenaid in patients with mild Alzheimer’s disease: Results of multi-center, 24-week, open-label extension study. Journal of Alzheimer’s Disease 2015; 44(2), 471-480.
